Groundbreaking Study reveals a 76% drop in hospital readmissions and a 41% decrease in Emergency Department visits, underscoring SHL and SmartHeart®’s pivotal role in remote cardiac care
SHL Telemedicine Ltd. (NASDAQ: SHLT, SIX: SHLTN;) ("SHL" or the "Company"), a leading provider and developer of advanced personal telemedicine solutions, is excited to announce the groundbreaking full results of the Imperial College London TELE-ACS Trial. The randomized clinical trial showcased, among other things, how SHL’s SmartHeart® 12-lead ECG technology can significantly reduce hospital readmissions and ED visits for post-MI (heart attack) patients at home.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240410735175/en/
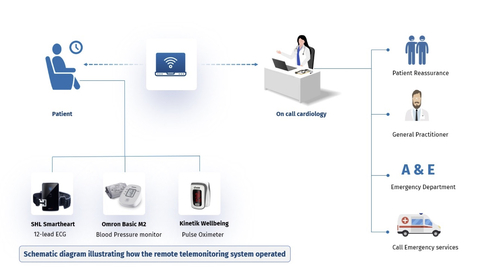
Photo: Visual Abstract from TELE-ACS trial presentation at ACC24 (Graphic: Business Wire)
Key Findings from the Study:
- A 76% reduction in the likelihood of hospital readmission within six months for patients using telemedicine.
- A 41% decrease in the likelihood of attending the emergency department (ED) compared to standard care recipients.
- Significant reductions in unplanned coronary revascularizations.
- Notable decreases in patient-reported symptoms, including chest pain, breathlessness, and dizziness.
The TELE-ACS clinical trial, conducted at a large tertiary center in London, UK, from January 2022 to April 2023, involved 337 participants. It has set a new benchmark in the use of telemedicine for the management of post-ACS patients by reducing hospital readmissions, ED visits, lowering unplanned revascularization rates and improving patient outcomes. The investigator initiated trial results, led by Dr. Ramzi Khamis and Nasser S. Alshahrani, were presented at the prestigious American College of Cardiology's 24th Annual Scientific Session & Expo Late-Breaking Clinical Trials Sessions (ACC 24 LBCT) in Atlanta. Moreover, the study was also published in the leading Journal of the American College of Cardiology (JACC).
Erez Nachtomy, CEO of SHL Telemedicine, commented on the significance of these results, stating: "The results of this pivotal study alongside their presentation at ACC 24, and publication in JACC, serve as a resounding vote of confidence in SHL and our SmartHeart® platform as a transformative technology for cardiac care and telemedicine as a whole. These groundbreaking clinical results are an endorsement that propels us forward in our mission to redefine patient care, demonstrating the tangible benefits and efficiency of our telemedicine solutions on a global scale."
For further Information:
- ACC24 Presentation Details: https://www.acc.org/Latest-in-Cardiology/Articles/2024/04/02/17/02/sat-415pm-tele-acs-acc-2024
- JACC Abstract Publication: https://www.jacc.org/doi/10.1016/j.jacc.2024.03.398
About SHL Telemedicine
SHL Telemedicine is engaged in developing and marketing personal telemedicine systems and the provision of medical call center services, with a focus on cardiovascular and related diseases, to end users and to the healthcare community. SHL Telemedicine offers its services and personal telemedicine devices to subscribers utilizing telephonic and Internet communication technology. SHL is listed on the SIX Swiss Exchange (SHLTN, ISIN: IL0010855885, Security No.: 1128957) and on the Nasdaq Stock Exchange (SHLT, ISIN: US78423T2006, CUSIP: 78423T200).
For more information, please visit our website at www.shl-telemedicine.com.
Forward-Looking Statements
Some of the information contained in this press release contains forward-looking statements. Readers are cautioned that any such forward-looking statements are not guarantees of future performance and involve risks and uncertainties, and that actual results may differ materially from those in the forward-looking statements as a result of various factors. SHL Telemedicine undertakes no obligation to publicly update or revise any forward-looking statements.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240410735175/en/
Contacts
Fabienne Farner, IRF, Phone : +41 43 244 81 42, farner@irf-reputation.ch





